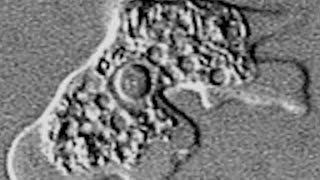
IMPAVIDO NOW AVAILABLE IN THE USA
Impavido (miltefosine) is now available in the USA directly from Profounda for the treatment of leishmania.

Profounda takes on Leishmaniasis
Profounda licenses Impavido from Knight Therapeutics for marketing into the US. Leishmaniasis is a parasitic disease that is found in...


Knight Profounda U.S. Partner for Impavido
MONTREAL, Canada, September 25, 2015 – Knight Therapeutics Inc. (TSX: GUD) (“Knight”), through one of its wholly-owned subsidiaries,...


Profounda to attend CCA Meeting In Orlando on August 6/7th 2015
Profounda will be introducing Rhinase Soothing Nasal Mist and Lubricating Nasal Gel at the Convenient Care Association Meeting held in...


Fall Relief to Allergies on its way
Profounda Plans to launch Rhinase in Fall 2015 Profounda Inc. (private) today announced the pre-market preparation for the planned launch...


Profounda licenses 2 OTC products for US market
Profounda Inc. (private) today announced the signing of a license agreement with a major pharmaceutical company for a line of OTC...


Profounda moves to new corporate offices in Orlando, FL
Starting September 1st, Profounda will be located at 5790 Hoffner Avenue, Suite 507, Orlando, Florida 32822-4824


Profounda receives additional milestone to further develop products
Profounda received additional private equity funding for development of Profounda's products. Profounda works with third party vendors...


Profounda completes Series A Fund raising
Profounda completes series A2 round of financing which will allow for future development of its pipeline products. new specialty...


Profounda OutLicenses right to development product
Profounda completes ex-US licensing outlicensing rights for a product in development in exchange for upfront milestone and downstream...